On April 17, juniors and seniors in the Madia Department of Chemistry, Biochemistry, and Physics who are enrolled in Nate McElroy’s CHEM 403 Advanced Chemistry Lab were the first IUP students to participate in a blood testing experiment to screen for organic toxins.
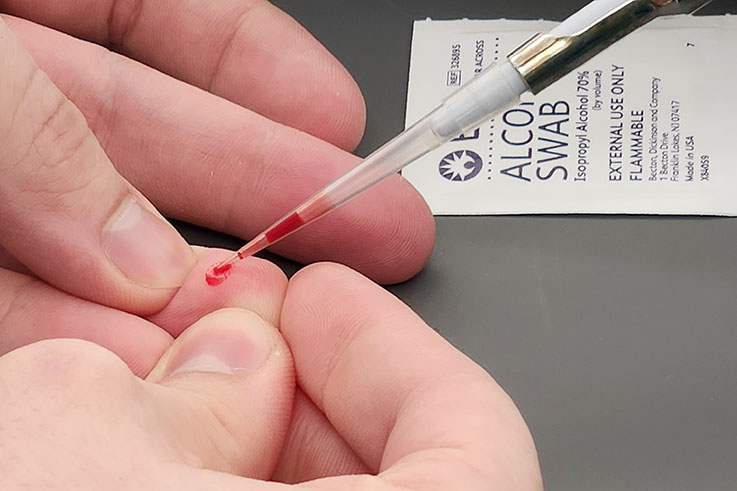
Working in pairs, one student served as the subject while the other student took the blood sample. The subject used a lancet to prick their fingertip while the analyst gathered 100 microliters (approximately 0.1 grams) of blood and placed it in a small vial.
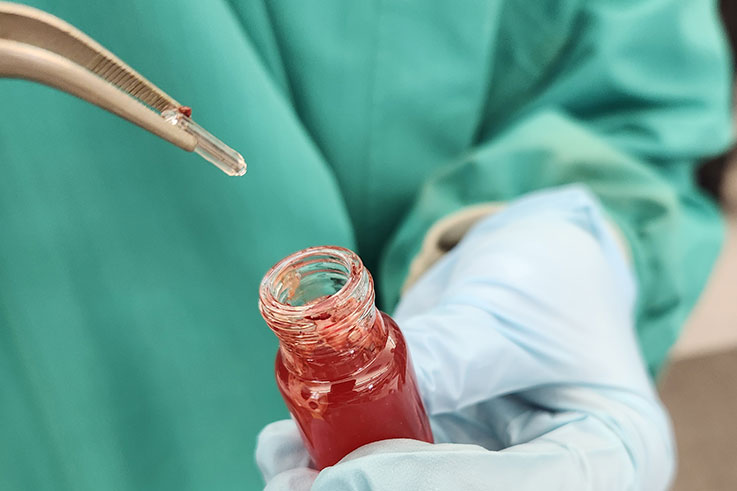
The vial with the blood sample was then “spiked” with a known ratio of heavy isotopes of each of the 21 POPs, sterilized with acetonitrile, and diluted with water. A specially coated magnetic stir bar was placed into the vial, and the sample was stirred vigorously for one hour. The stir bar (Twister™) is coated with a hydrophobic polymer (polydimethylsiloxane), to which any organic compound in the blood adheres. After an hour’s exposure, the stir bar was removed, rinsed, dried, and placed into a special glass insert for further processing in the department’s gas chromatography mass spectrometry (GC/MS) facility.

Howard ‘Skip’ Kingston (BS’73, M’75) and Jeremiah Jamron, who both relocated to IUP from Duquesne University as scholars in residence in 2023, assisted students with the collection and processing of blood samples. Kingston’s research is centered around isotope dilution mass spectrometry (IDMS), which allows for much more accuracy and sensitivity than traditional mass spec methods.

After completion of the lab, Kingston and Jamron collected all samples for analysis using IDMS. Each student stir bar was processed by a Gerstel robotic system attached to an Agilent GC/MS in a “clean room” environment that avoids contaminants from the ambient lab air. Each stir bar was thermally desorbed and then exposed briefly to liquid nitrogen to concentrate the organic components, followed by flash heating to off-gas toxins into the instrument. All organic compounds, including the POPs, were then analyzed by the GC/MS.
Results were returned to each student with a spreadsheet that would allow them to calculate the concentration of any POP found in their blood sample. Of the nine students whose blood was analyzed, all students had measurable levels of:
- Bis(2-ethylhexyl)phthalate (DEHP) – a widely-used plasticizer used in PVC pipes, food packaging, water bottles, and consumer goods
- Naphthalene – the key ingredient in mothballs, it is also used in the production of dyes, plastics, resins, and other chemicals
- Toluene – a common solvent in paints, adhesives, and gasoline
- Xylene – a common solvent in paints, varnishes, rubber and leather industries, and gasoline
- 2,2’,4,4’,5-pentachlorobiphenyl (PCB-099) – once used in heat transfer applications, insulation, and additives in plastics, PCBs were banned in the US in the 1970s
- 4,4’-dichlorodiphenyltricholorethane (DDT) – a once widely used insecticide, banned in the US in 1972, though still used in other countries in Asia, Africa, and South America
This small sampling of students’ results is typical of similar recent studies in the US and UK. Persistent organic pollutants, including PCBs and DDT, “forever chemicals” such as per- and polyfluoroalkyl substances (PFAS), and other pollutants are commonplace in our world—from the air, our water sources, our foods, and any number of consumer products we’re exposed to on a daily basis.
Kingston and his collaborators are working on methods now that will allow anyone to have their blood analyzed for POPs and heavy metals, regardless of their location or access to healthcare.